- Published on
Revolutionizing Medical Device Development with a Hybrid Agile-Waterfall Approach
- Authors

- Name
- Venkat Venkatakrishnan
Revolutionizing Medical Device Development with a Hybrid Agile-Waterfall Approach
Introduction
In the rapidly evolving field of medical device manufacturing, the integration of innovative product development processes remains a cornerstone for success. This white paper explores a pioneering approach adopted for a Fortune 500 client in the medical device sector, detailing a hybrid Software Development Life Cycle (SDLC) that aligns with global standards such as FDA, EU regulations, and ISO 13485.
The Challenge
The client, a leader in medical technology, faced challenges in adapting to the fast-paced changes in technology while ensuring compliance with stringent international standards. The existing development processes were old and rigid, slowing down innovation and adaptation to market needs. The client also needed the flexibility to incorporate AI/ML technologies in its product.
Innovative Approach to Product Development
The newly developed process integrates three unique features to overcome these challenges:
A. Hybrid Agile-Waterfall SDLC: Combining the flexibility of Agile with the structured approach of Waterfall, this hybrid model facilitates rapid development while maintaining rigorous documentation and compliance necessary for medical device approval. The process also enables the product team to manage AI/ML algorithms in its sprints, including its training and validation.
B. Cloud-Based Architecture: Transitioning from on-site servers to a cloud-based solution ensured scalability, security, and compliance. The migration included thorough validation of the cloud architecture, ensuring it met all regulatory requirements for medical software. Further, this provides necessary infrastructure to spin up special servers to develop and train new algorithms.
C. Reduction in Paper Requirements: By optimizing and digitizing documentation processes, the new system reduced paper requirements by 80%, streamlining operations and reducing overhead.
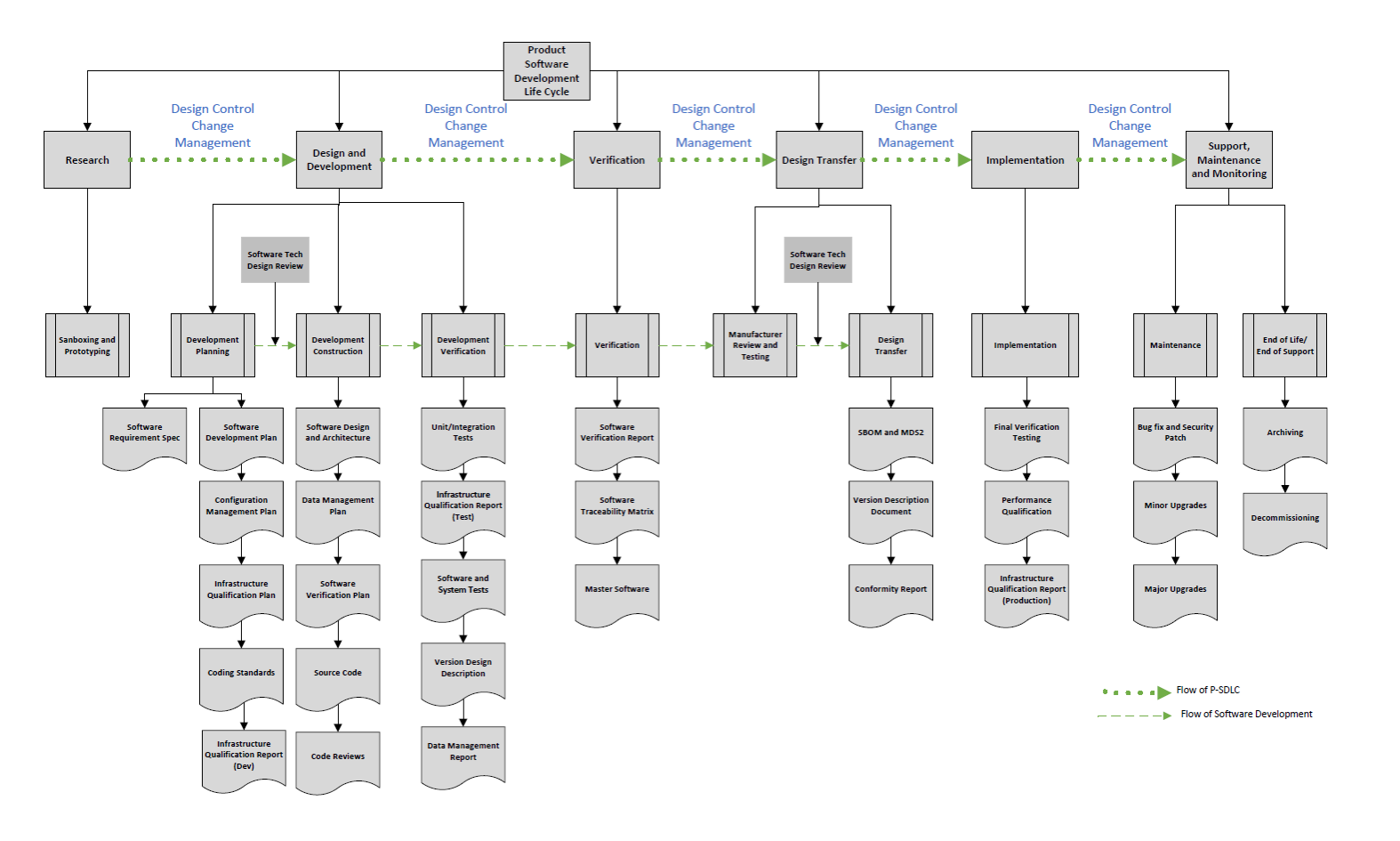
Implementation and Training
The implementation of this new system was comprehensive, requiring extensive training for over 500 employees across five countries. The training program was meticulously developed to ensure minimal disruption to day-to-day operations, equipping staff with the necessary skills to leverage the new system effectively.
Conclusion
This case study exemplifies the potential of innovative approaches in the development of medical devices. The hybrid SDLC model not only enhances efficiency but also ensures compliance with international standards, representing a significant leap forward in medical device development.
Future Directions
The success of this project serves as a model for similar initiatives, promising substantial improvements in the development and compliance of medical devices worldwide. This white paper will serve as a critical resource for organizations aiming to refine their development processes in line with contemporary technological and regulatory demands.